Number Of Valence Electrons In Zinc
I’m now going to demonstrate how to bond Beryllium Hydride and what is its molecular geometry…

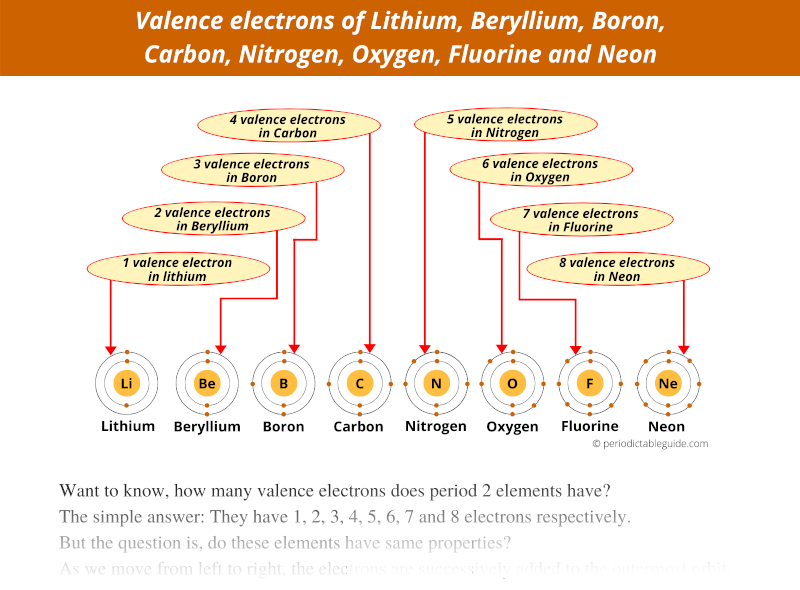

The Number Of Valence Electrons In Beryllium
- Beryllium has two valence electrons. Valence electrons are those electrons that are capable of participating in the formation of chemical bonds with other atoms. These chemical bonds occur through the sharing of these two electrons with other atoms. Valence electrons get their name from their location within an atom.
- Sunday, August 04, 2019 A valence electron is an outer shell electron and may participate in the formation of a chemical bond. Ok but how many valence electrons does an atom of Beryllium have? In the case of Beryllium the valence electrons is 2.
As shown in the image above we begin this process by writing the formula of the molecule. In this case our formula is BeH2. After we write the formula down we use a periodic table or our previous knowledge on the amount of valence electrons for the elements we use. For this problem Be has 2 valence electrons and each H has 1 valence electron. We already know that a bond between molecules is created by 2 electrons. Therefore, we can create 2 bonds, one on each side of Be, as Be is our central atom. Once we have formed the bonds we check for the octet rule or otherwise known as the rule of 8. Since BeH2 does not have enough electrons around it to satisfy the rule of 8 we simply say that it does not satisfy it. If you want you may check the number of bonds that you can make with the application of the number of bonds chart. The chart is divided into 3 columns and 3 rows. In the first column we write the elements we are using. In the second column we write the number of electrons that are needed for the element to be “happy” or complete for each element. In the third column we write the number of electrons that we do have for each element. We add up all of the numbers from each column corresponding to their designated column. In this case the use of the chart is unnecessary as we do not have enough electrons present. The next step is to set up the Lewis Structure of the molecule. According to our structure we can say that the shape of our molecule is Linear.
A 3D image of the shape is given below.
Oct 11, 2018 The number of dots equals the number of valence electrons in the atom. The element beryllium has atomic number 4 and appears in the Group 2 column of the periodic table. It has two electrons in its valence shell (like all the other Group 2 elements), and we write it by using its chemical symbol (Be) and two dots like this: Be.File:Lewis dot.
