What is the mass number of oxygen?
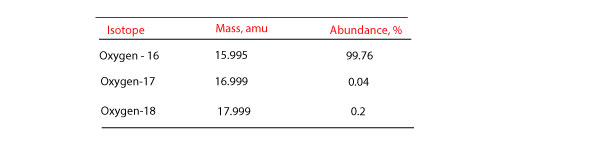
What is the percentage abundance of the other two isotopes, using the average atomic mass of 15.9994 u. Socratic Oxygen is composed of three isotopes 16/8 O (15.995 u), 17/8 O (16.999 u) and 18/8 O (17.999 u). One of these isotopes, 17/8 O, comprises of 0.037% of oxygen. Calculate the atomic mass of oxygen if the three common isotopes of oxygen have masses of 15.995 amu (99.759% abundance), 16.995 amu (0.037% abundance), and 17.999 amu (0.204% abundance). (please show work) I will give brainliest answer! The chemists resisted making the amu one-sixteenth the mass of an oxygen-16 atom; it would change their atomic weights by about 275 parts per million. Making the amu one-twelfth the mass of a carbon-12 nucleus, however, would lead to only a 42 parts per million change, which seemed within reason.
2 Answers
The most common Isotope Mass for Oxygen is 16 Atomic Mass Units.
Explanation:
The Weighted Average Atomic Mass for each element is found on the periodic table (That long decimal number near the bottom of each box). This number is a combination of all the known Isotopes and basically tells you the average mass you would expect per atom if you grabbed a random handful of them.
The Mass Number is the mass of a particular atom individually and is measured in Atomic Mass Units (AMU); these will always be whole numbers.
The most common Isotope is found by rounding the Weighted average found on the periodic table to the nearest whole number.
In this case Oxygen has a Weighted Average Atomic Mass of 15.999 AMU. So the Mass Number is 16.
Explanation:
Mass number
The most common isotope of oxygen has 8 neutrons. Its atomic number is 8.
This isotope of oxygen is oxygen-16.
Another isotope of oxygen has 9 neutrons.
This isotope is oxygen-17.
A third oxygen isotope has 10 neutrons.
This oxygen isotope is oxygen-18.
The following is the isotopic or nuclear notation of an isotope.
Related questions
Learning Objectives
- To determine the formula mass of an ionic or molecular compound.
A necessary skill for future chapters is the ability to determine the mass of the formula of an ionic compound. This quantity is called the formula mass. The formula mass is obtained by adding the masses of each individual atom in the formula of the compound. Because a proper formula is electrically neutral (with no net electrons gained or lost), the ions can be considered atoms for the purpose of calculating the formula mass.
Let us start by calculating the formula mass of sodium chloride (NaCl). This formula mass is the sum of the atomic masses of one sodium atom and one chlorine atom, which we find from the periodic table; here, we use the masses to two decimal places:
Na: 22.99 amu
Cl: +35.34 amu
Total: 58.44 amu
To two decimal places, the formula mass of NaCl is 58.44 amu.
When an ionic compound has more than one anion or cation, you must remember to use the proper multiple of the atomic mass for the element in question. For the formula mass of calcium fluoride (CaF2), we must multiply the mass of the fluorine atom by 2 to account for the two fluorine atoms in the chemical formula:
Ca: 1 x 40.08 = 40.08 amu
F: 2 x 19.00 = +38.00 amu
Total = 78.08 amu
The formula mass of CaF2 is 78.08 amu.
For ionic compounds with polyatomic ions, the sum must include the number and mass of each atom in the formula for the polyatomic ion. For example, potassium nitrate (KNO3) has one potassium atom, one nitrogen atom, and three oxygen atoms:
K: 1 x 39.10 = 39.10 amu
N: 1 x 14.00 = +14.00 amu
O: 3 x 16.00 = +48.00 amu
Total = 101.10 amu
The formula mass of KNO3 is 101.10 amu.
Potassium nitrate is a key ingredient in gunpowder and has been used clinically as a diuretic.
When a formula contains more than one polyatomic unit in the chemical formula, as in Ca(NO3)2, do not forget to multiply the atomic mass of every atom inside of the parentheses by the subscript outside of the parentheses. This is necessary because the subscript refers to the entire polyatomic ion. Thus, for Ca(NO3)2, the subscript 2 implies two complete nitrate ions, so we must sum the masses of two (1 × 2) nitrogen atoms and six (3 × 2) oxygen atoms, along with the mass of a single calcium atom:
Ca: 1 x 40.08 = 40.08 amu
N: 2 x 14.00 = +28.00 amu
Oxygen Isotopes Amu
O: 6 x 16.00 = +96.00 amu
Total = 164.08 amu
The key to calculating the formula mass of an ionic compound is to correctly count each atom in the formula and multiply the atomic masses of its atoms accordingly.
Example (PageIndex{1})
Use the atomic masses (rounded to two decimal places) to determine the formula mass for each ionic compound.
- FeCl3
- (NH4)3PO4
Solution
a.
Fe: 1 x 55.85 = 55.85 amu
Cl: 1 x 35.45 = +106.35 amu
________________________
Total = 162.20 amu
The formula mass of FeCl3 is 162.2 amu.
b. When we distribute the subscript 3 through the parentheses containing the formula for the ammonium ion, we see that we have 3 nitrogen atoms and 12 hydrogen atoms. Thus, we set up the sum as follows:
N: 3 x 14.00 = 42.00 amu
H: 12 x 1.00 = +12.00 amu
P: 1 x 30.97 = +30.97 amu
O: 4 x 16.00 = +64.00 amu

Total = 148.97 amu
Ambulatory Oxygen
The formula mass for (NH4)3PO4 is 149.0 amu.
Exercise (PageIndex{1})
Amulet Oxygen
Use the atomic masses (rounded to two decimal places) to determine the formula mass for each ionic compound.
- TiO2
- AgBr
- Au(NO3)3
- Fe3(PO4)2
Answer
a. 79.87 amu

b. 187.77 amu
c. 383.0 amu
To Your Health: Hydrates
Some ionic compounds have water (H2O) incorporated within their formula unit. These compounds, called hydrates, have a characteristic number of water units associated with each formula unit of the compound. Hydrates are solids, not liquids or solutions, despite the water they contain.
To write the chemical formula of a hydrate, write the number of water units per formula unit of compound after its chemical formula. The two chemical formulas are separated by a vertically centered dot. The hydrate of copper(II) sulfate has five water units associated with each formula unit, so it is written as CuSO4•5H2O. The name of this compound is copper(II) sulfate pentahydrate, with the penta- prefix indicating the presence of five water units per formula unit of copper(II) sulfate.
Hydrates have various uses in the health industry. Calcium sulfate hemihydrate (CaSO4•½H2O), known as plaster of Paris, is used to make casts for broken bones. Epsom salt (MgSO4•7H2O) is used as a bathing salt and a laxative. Aluminum chloride hexahydrate is an active ingredient in antiperspirants. The accompanying table lists some useful hydrates.
| Formula | Name | Uses |
|---|---|---|
| AlCl3•6H2O | aluminum chloride hexahydrate | antiperspirant |
| CaSO4•½H2O | calcium sulfate hemihydrate (plaster of Paris) | casts (for broken bones and castings) |
| CaSO4•2H2O | calcium sulfate dihydrate (gypsum) | drywall component |
| CoCl2•6H2O | cobalt(II) chloride hexahydrate | drying agent, humidity indicator |
| CuSO4•5H2O | copper(II) sulfate pentahydrate | fungicide, algicide, herbicide |
| MgSO4•7H2O | magnesium sulfate heptahydrate (Epsom salts) | laxative, bathing salt |
| Na2CO3•10H2O | sodium carbonate decahydrate (washing soda) | laundry additive/cleaner |
Key Takeaway
- Formula masses of ionic compounds can be determined from the masses of the atoms in their formulas.
Oxygen Amu Mass

Contributors and Attributions
Anonymous
Marisa Alviar-Agnew (Sacramento City College)
Henry Agnew (UC Davis)
